Our Science
Impact of LRRK2-I1371V mutation on BM-MSCs and their interactions with astroglial cells in PD
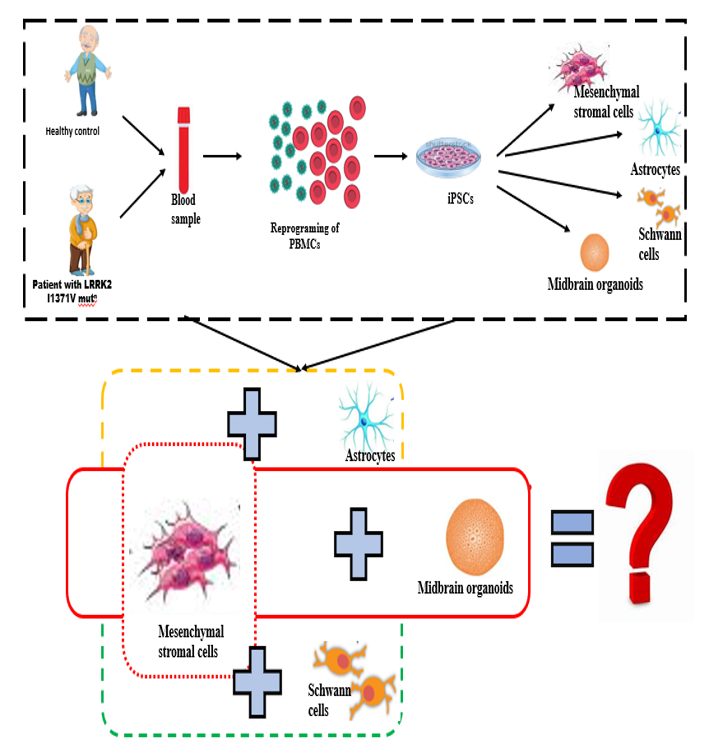
Mutations in LRRK2 gene are implicated in both familial and sporadic forms of PD. The LRRK2 gene contains kinase and GTPase domains, with mutations like G2019S and I1371V affecting these domains, and influencing disease risk, severity, and treatment responses. Peripheral inflammation and immune dysfunction, including increased microglial activation and pro-inflammatory cytokines, are key hallmarks of PD progression. LRRK2 mutations are associated with immunological alterations and enhanced inflammatory responses .In PD, mesenchymal stromal cells (BMMSCs), which typically regulate inflammation, become dysfunctional, leading to persistent inflammation. LRRK2 is involved in immune functions like phagocytosis and antigen presentation, but the impact of the I1371V mutation on BMMSCs and their interactions with astrocytes and Schwann cells is not well understood. This study explores how the I1371V mutation affects MSCs and their interactions with astrocytes, Schwann cells and 3D midbrain organoids, using patient-derived iPSCs, to better understand PD mechanisms and inform potential therapeutic strategies.
Role of BM-MSCs in the Early PD Neuroinflammatory Milieu
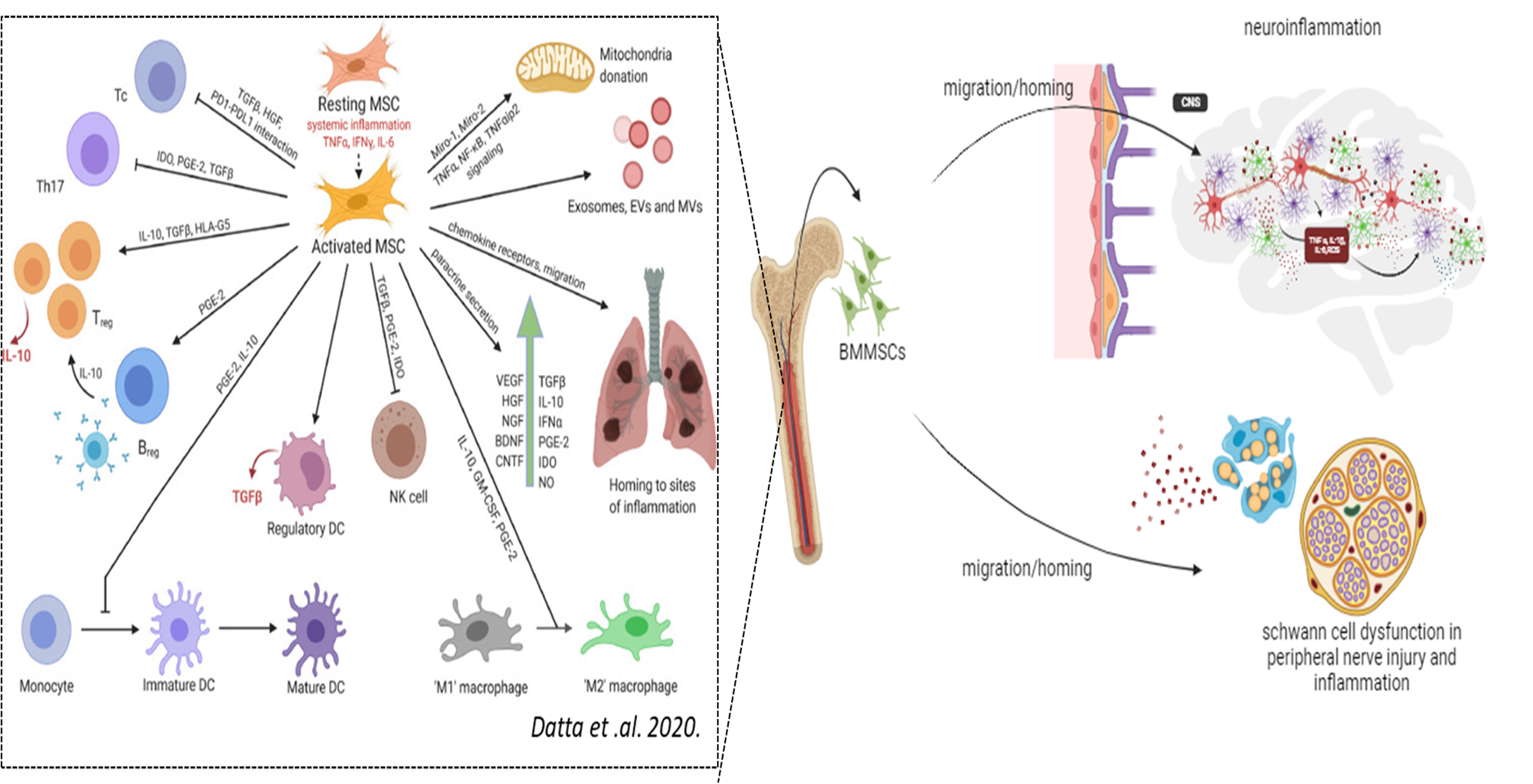
Under a physiological inflammatory milieu, Bone Marrow Mesenchymal Stromal Cells (BMMSCs) are activated and play a crucial regulatory role in modulating inflammation while also exhibiting neuroprotective and regenerative functions. Failure of this immunomodulation can lead to a continuous cycle of systemic and neuronal inflammation. In Parkinson’s disease (PD) of genetic or sporadic origin, chronic inflammatory conditions are observed in both the central and peripheral nervous systems, driven by the activation of neuronal and peripheral glial cells. Our research focuses on investigating the physiology of mesenchymal stromal cells and their interaction with glial cells during the early stages of PD. This work aims to enhance the development of more effective MSC transplantation therapies for PD.
Experimental Validation of Disease Pathology in relevant cell-types differentiated from patient-derived iPSCs carrying PLA2G6 mutations associated with neurodegeneration
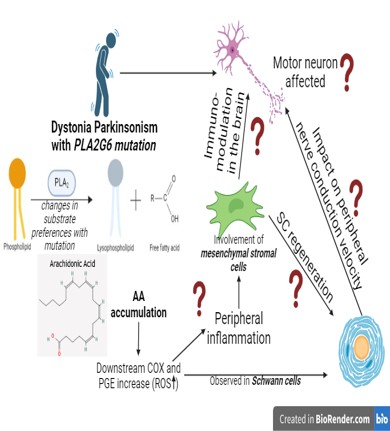
Mutations in the Phospholipase A2 group VI (PLA2G6) gene have been identified in dystonia parkinsonism (referred to as “PLA2G6 mutations associated with neurodegeneration” or PLAN), where there may be modifications in the substrate preferences or regulatory mechanisms for PLA2G6 (such as changes in the binding to calmodulin or other proteins), or associated with inhibition of the phospholipid-hydrolysing functions of PLA2G6. Clinical findings from PLAN suggest that the mutation may impact cell-types other than dopaminergic (DA) neurons, such as Schwann cells and bone marrow immunomodulatory cells. Oxidative stress (due to arachidonic acid (AA) accumulation and its metabolism) is considered a key pathophysiological mechanism underlying DA neuronal loss in Parkinson’s disease (PD). The inflammatory metabolites released from AA metabolism are also observed in Schwann cells in PD post-mortem data. Further, the release of potent mediators of inflammation such as prostaglandins, prostacyclin and thromboxane in the disease state necessitates immunomodulation by bone-marrow mesenchymal stromal cells (BM-MSCs). BMMSCs are recognized mediators of Schwann cell regeneration for myelination of the sciatic nerve, deficiencies in which eventually affects nerve conduction velocity and gait. We thus intend to look into how the PLA2G6 mutation affects cellular functions and disease pathology in DA neurons, Schwann cells and BM-MSCs, leading to the PD phenotype.
Role of phosphorylated α-synuclein in dopaminergic neuron dysfunction
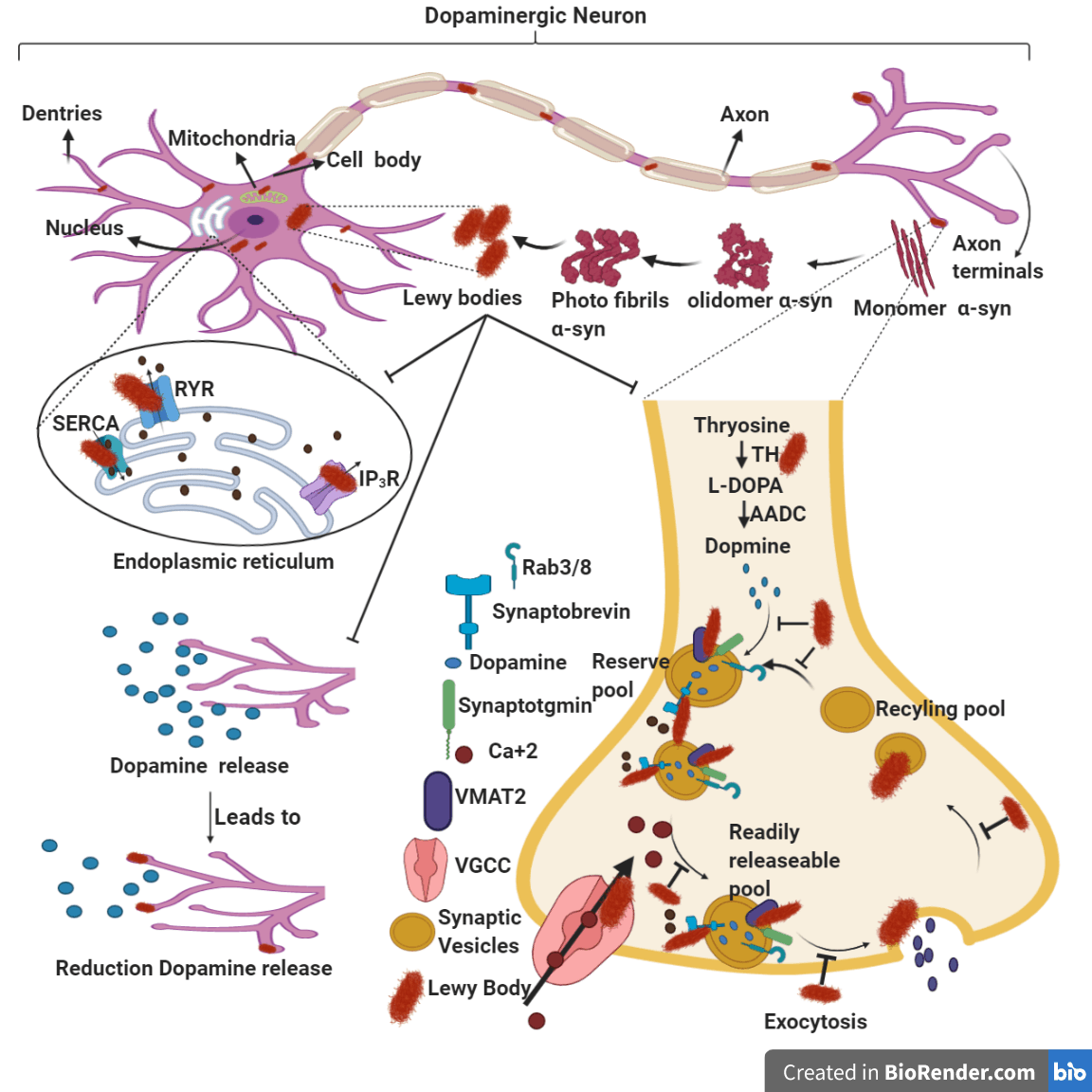
Among the several post-translational modifications of α-synuclein that are known to occur in Parkinson’s disease, phosphorylation at the serine 129 region is the most common, leading to the accumulation of Lewy Bodies that is a pathological hallmark of PD. Approximately 90% of the α-synuclein deposited in Lewy Bodies is extensively phosphorylated at the serine 129 region, compared to only 4% or less in a normal healthy brain. The key kinases involved in the phosphorylation of α-synuclein are POLO-Like kinase (PLK), Casein Kinase2 (CK2), Leucine Rich Repeat Kinase 2 (LRRK2) and G-Protein Coupled Receptor Kinase 5 (GRK5). Inhibition of these individual kinases through siRNA has been shown to decrease phosphorylation of α-synuclein in WT and A53T α-synuclein transfected SH-SY5Y cells. While there are studies indicating an association between α-synuclein aggregation and reduction in dopamine release, the importance of α-synuclein phosphorylation to the process of vesicular dopamine release, their exocytosis and recycling, along with changes in intracellular calcium upon physiological stimulation and Store-Operated Ca2+ Entry (SOCE) are unreported. This is now under investigation.
Evaluation of LRRK2 I1371V mutation on the cellular pathogenesis of Parkinson’s diease using induced pluripotent stem cells
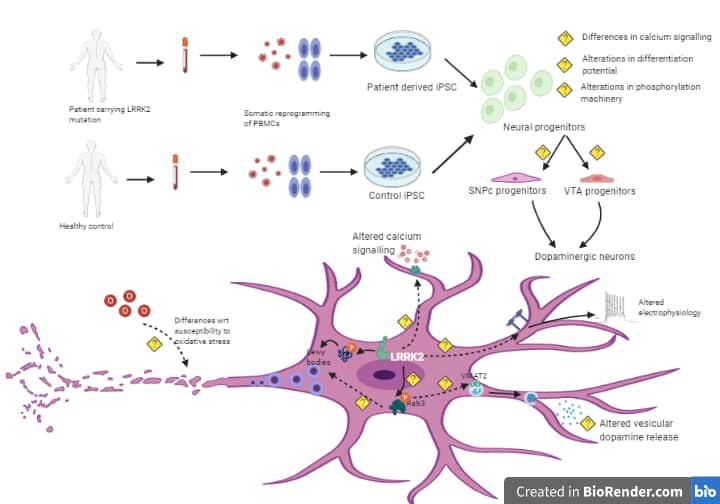
Mutations in the LRRK2 gene are implicated in both familial and sporadic forms of PD. The LRRK2 gene contains kinase and GTPase domains, with mutations like G2019S and I1371V affecting these domains, and influencing disease risk, severity, and treatment responses. Peripheral inflammation and immune dysfunction, including increased microglial activation and pro-inflammatory cytokines, are key hallmarks of PD progression. G2019S and other LRRK2 mutations are associated with immunological alterations and enhanced inflammatory responses .In PD, mesenchymal stromal cells (BMMSCs), which typically regulate inflammation, become dysfunctional, leading to persistent inflammation. LRRK2 is involved in immune functions like phagocytosis and antigen presentation, but the impact of the I1371V mutation on BMMSCs and their interactions with astrocytes and Schwann cells is not well understood. This study is exploring how the I1371V mutation affects MSCs and their interactions with astrocytes, Schwann cells and 3D midbrain organoids, using patient-derived iPSCs, to better understand PD mechanisms and inform potential therapeutic strategies.
Parkinson’s disease pathogenesis by astrocytes: differential response to different forms of extracellular α-synuclein
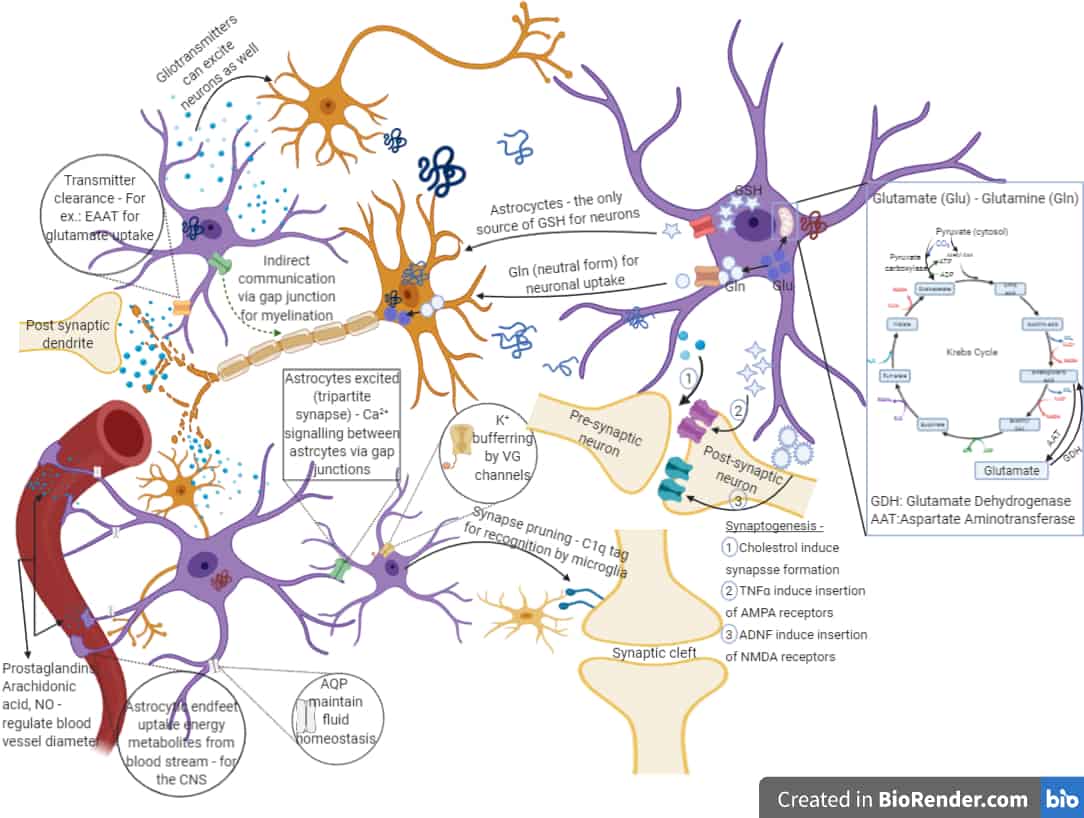
Our work focuses on the pathology caused by astrocytes due to their association with wild type or mutated forms of α-synuclein. Many in vivo and post-mortem studies have indicated the presence of monomeric and/or aggregated species of α-synuclein in dystrophic astrocytes, suggesting the deterioration of the cellular niche (astrocytes) that paves the way for neurodegeneration. However, the cellular and molecular mechanisms behind this is yet to be elucidated. Dysfunction of astrocytes in terms of defence against oxidative stress and other stress-indicative markers, glutamate clearance, calcium dynamics in presence of physiological stimulation, ER and lysosomal impairments, accumulation of other modified species of α-synuclein and the crosstalk between affected astrocytes and dopaminergic neurons – these are a few of the topics addressed in our research. We hope this understanding will help us design novel therapeutic strategies targeting the niche cells.
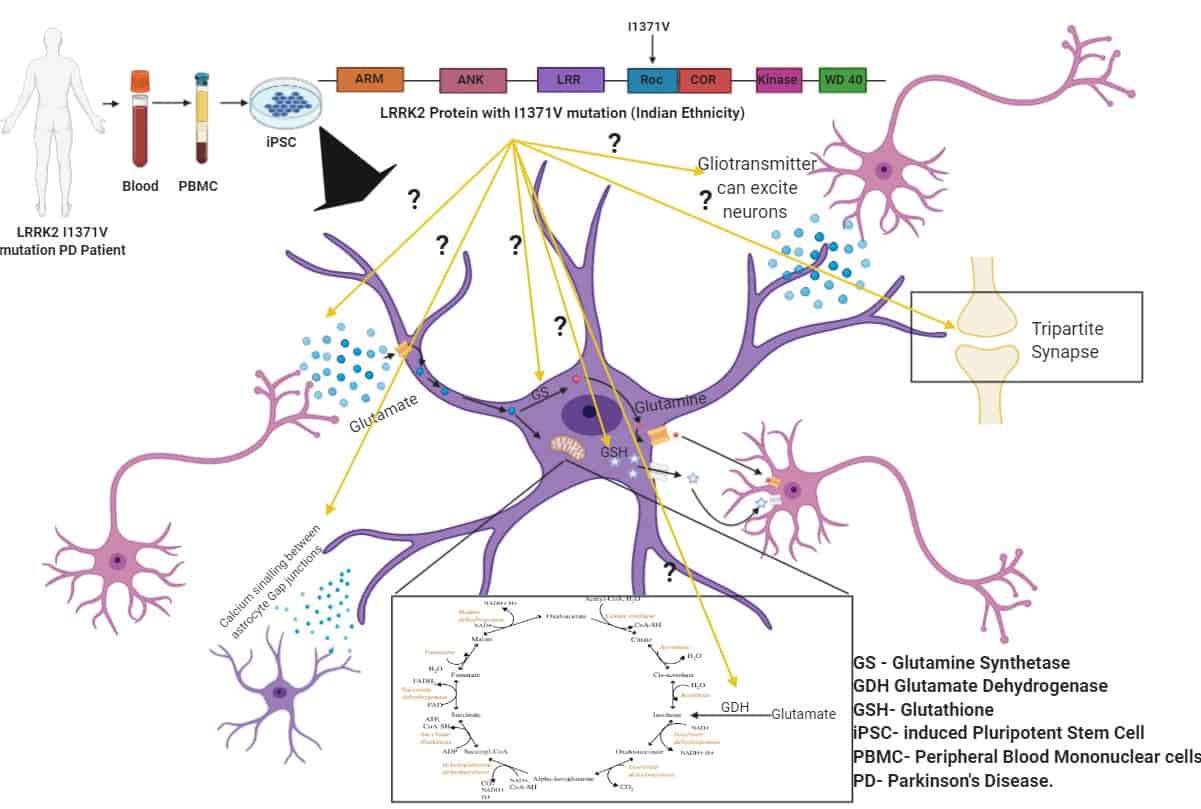
Astrocytes derived from Indian Ethnicity LRRK2 I1371V mutation Patient-Specific iPSCs and their non-cell autonomous role on the neurodegeneration of Parkinson’s disease.
Astrocytes, the structural support cells of the brain, also play a crucial role in the survival and functionality of neuronal cells through metabolic delivery of nutrients and/or anti-oxidants under physiological conditions. They are further capable of responding to pathological stimuli and consequently bring about changes in neuronal cells through neuron-glia crosstalk. These glial cells are now emerging as critical players in the pathogenesis of Parkinson’s disease. Among the many genes associated with PD is LRRK2, which represents the most common cause of inherited or familial PD. Several pathogenic missense LRRK2 mutations have been identified, the locations of which lie in different functional domains of LRRK2. While the G2019S mutation has been studied the most, the dysfunction or the morphological changes of the astrocytes caused due to the LRRK2 I1371V mutation (prevalent in India) is our subject of focus. We are evaluating the effect of the LRRK2 I1371V mutation on astrocytic fate commitment, cell vulnerability, cellular deregulation in reference to intracellular calcium response, lysosomal degradation, and the astrocytes’ neuroprotective functions on dopaminergic neurons.
Human Dental Pulp Stem Cells in the treatment of Diabetic Neuropathy: Does exogenous stem cell transplantation correct endogenous stem cell dysfunction?

Indications of endogenous Bone Marrow Mesenchymal Stem Cell (BM-MSC) dysfunction in diabetes have surfaced of late. Impaired cellular and molecular interactions between vascular and neurological components form the basis of disease progression in Diabetic Neuropathy. Our lab focuses on investigating BM-MSC dysfunction in pre-DN and post-DN rats, particularly with regard to their migratory/homing properties toward chemokines and cytokines, and the underlying intracellular calcium response. These experiments aid in answering some critical questions – does BM dysfunction precede the onset of neuropathy? Are endogenous DN-BMMSCs unable to protect DN-Schwann cells? And if BM-MSC dysfunction is indeed an early event, is that why chronic diabetic complications develop?
Biodistribution of transplanted human stem cells: Where do the cells go?
One of the biggest challenges in treatment strategies involving mesenchymal stem cell (MSC) therapy for any disease is determining the optimal route of transplantation and dosage. Key questions of how long MSCs dwell in the body and what tissues they migrate to need to be answered clearly so as to design the dose and frequency of MSC transplantations appropriately.
In our lab, we have tried to find answers to these questions by plotting the biodistribution of transplanted MSCs or its exosomes. Using minimally-invasive Near Infra-Red (NIR) whole animal and tissue imaging, we determine MSC/exosome retention time, course of movement, homing to niche and localization in organs.
The NIR range provides high sensitivity owing to least autofluorescence of animal tissue in this spectral region. In addition, it provides a higher depth of tissue penetration and therefore a higher signal-to-noise ratio. The technique requires minimal manipulation of the cells, and relies on simply tagging the plasma membrane with lipophilic NIR dyes that are quick, more stable, and do not require special handling.

Exosomes as a Drug Delivery tool
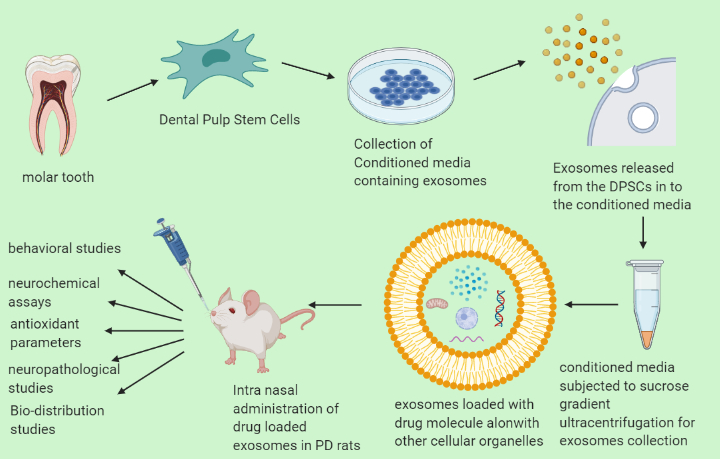
Exosomes are naturally occurring nano-sized vesicles produced by all cells, and range in size from 30-150nm. They carry the characteristics of their parent cell, have low immunogenicity, high bio-compatibility and minimum toxicity. DPSC-exosomes display similar neuro-modulatory and neuroprotective mechanism as DPSC themselves. Thus, they are an ideal delivery vesicle that crosses the BBB easily, unlike the cells they originate from. Being a neurodegenerative disorder, Parkinson’s Disease is difficult to treat as most of the drugs are administered as prodrugs that fail to provide the required concentration in the brain, owing to the BBB.
Exosomes are HLA-DR negative and bypass the mononuclear-phagocyte system to deliver their cargo without getting destroyed (Hall et al 2016).
Antioxidant-loaded exosomes delivered directly through the intranasal route maximize their availability in the midbain, avoiding the first pass metabolism and systemic distribution as observed in other routes of administration.